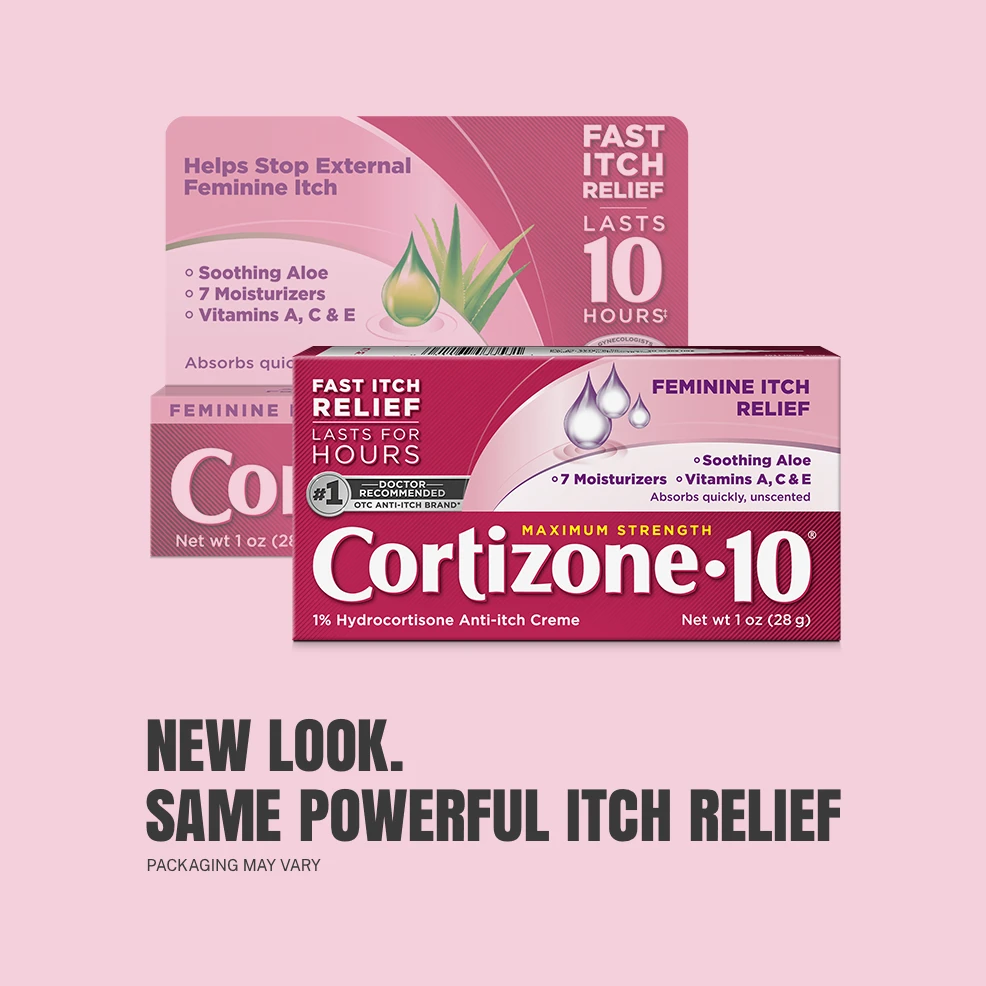
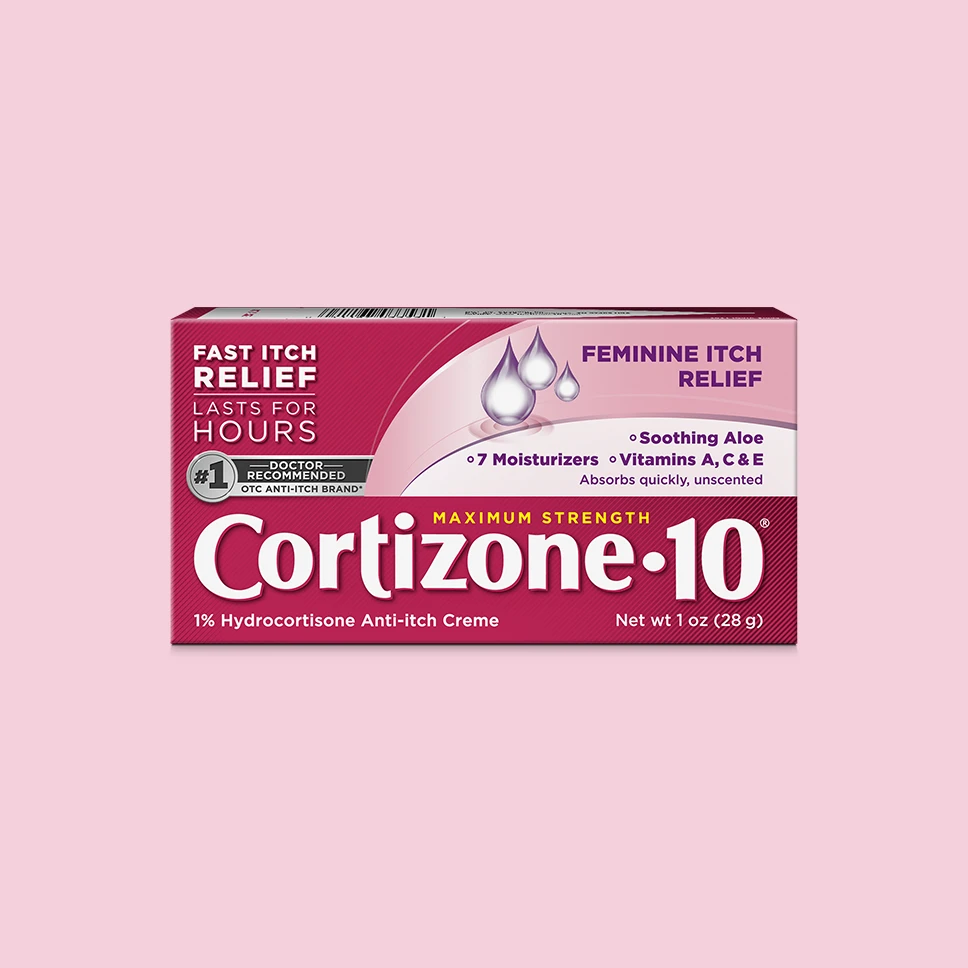
Cortizone-10® Maximum Strength
FEMININE ITCH RELIEF CREAM
Specifically formulated to provide fast, long-lasting itch relief for feminine itch.
Help relieve external feminine itching with Cortizone-10® Maximum Strength Itch Relief Feminine Itch Cream. With maximum-strength hydrocortisone available over the counter to help relieve itch in less than 10 minutes and provide relief that lasts for hours.
It includes seven moisturizers plus vitamins A, C, and E to nourish and hydrate your skin. It's formulated with the strongest non-prescription medicine* available for fast, lasting relief from feminine itch and safe for sensitive skin when used as directed.
Cortizone-10® is the #1 Doctor Recommended OTC Topical Anti-Itch Brand.†
Helps relieve itch fast
Long lasting
Soothing aloe vera
Fragrance free
Dye free
Gentle on skin
Moisturizes
Maximum-strength itch relief without a prescription
*Refers to the ingredient hydrocortisone.
*2024 IQVIA Study
Great for itch associated with:
Feminine itch
Sizes: 1 oz tube
WHERE TO BUY

FORMULA
HYDROCORTISONE 1%
Hydrocortisone is a steroid (corticosteroid) medicine that works to temporarily relieve itch and helps reduce inflammation. When skin is irritated a chemical reaction causes blood vessels to widen. After this, the skin can become red, swollen, and itchy. Topical hydrocortisone products like Cortizone-10® stop these reactions and temporarily reduce itching from minor skin irritations.
7 NOURISHING MOISTURIZERS
7 nourishing moisturizers work to attract, retain, and lock in moisture while hydrocortisone provides temporary itch relief that lasts for hours.
water, glycerin, dimethicone, petrolatum, jojoba esters, cetyl alcohol, aloe barbadensis leaf juice, stearyl alcohol, distearyldimonium chloride, cetearyl alcohol, steareth-2, steareth-21, chamomilla recutita (matricaria) flower extract, tocopheryl acetate, magnesium ascorbyl phosphate, retinyl palmitate, hydrolyzed jojoba esters, beta-glucan, glyceryl stearate, menthyl lactate, polysorbate 60, methyl gluceth-20, stearamidopropyl PG-dimonium chloride phosphate, PPG-12/SMDI copolymer, potassium hydroxide, diazolidinyl urea, propylene glycol, benzyl alcohol, methylparaben, BHT, propylparaben, EDTA

PRODUCT INFORMATION
- temporarily relieves external feminine itching
- other uses of this product should only be under the advice and supervision of a doctor
- when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly then gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product
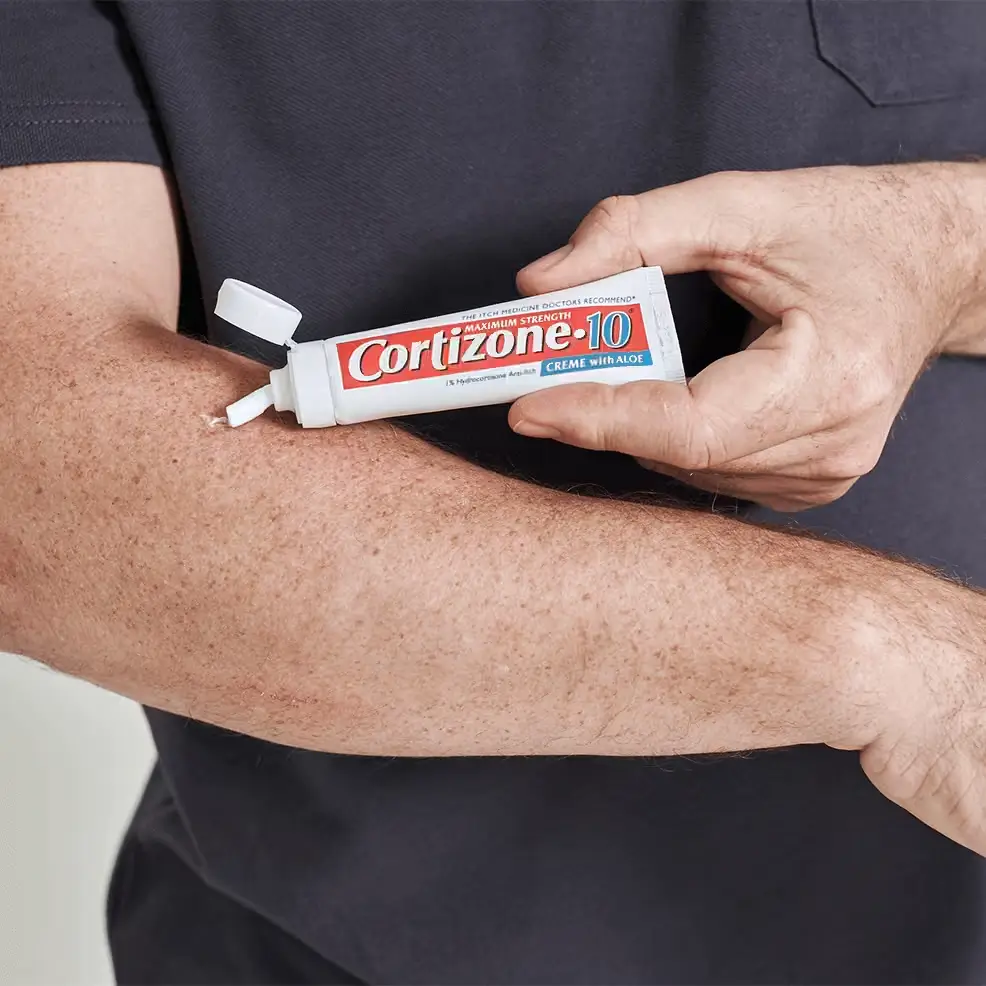
- adults and children 12 years of age and older: apply a fingertip amount (approximately 1-inch strip) to affected area not more than 3 to 4 times daily
- children under 12 years of age: ask a doctor
- for the treatment of diaper rash. Consult a doctor.
- if you have vaginal discharge. Consult a doctor.
- if you are allergic to any ingredient in this product
- avoid contact with eyes
- do not use more than directed unless told to do so by a doctor
For external use only
Do not useFREQUENTLY ASKED QUESTIONS
-
No, there’s no safety seal on the tube. Do not use if the carton has been tampered with or if the product appears to have been used. We recommend keeping the product out of the reach of children.
-
Store in a cool, dry place out of direct sunlight.
-
No, for your own safety expired products should not be used.
-
Consult your healthcare professional before using if you are pregnant or breastfeeding and ask if Cortizone-10® can help.
-
Cortizone-10® can be applied externally to your skin. Please refer to the Drug Facts for indicated uses. For additional questions, consult your healthcare professional and ask if Cortizone-10® can help.
-
Use as directed. Apply to the affected area.
-
The product is fragrance free. So you’ll know it’s there, but nobody else will.
-
The “10” refers to 1.0% hydrocortisone in our products.
-
No, Cortizone-10® products are not tested on animals.
-
You may be able to use your HSA and FSA tax-preferred savings to purchase certain OTC products, including Cortizone-10®. The passage of the CARES Act by Congress includes provisions to restore OTC eligibility under tax-preferred HSAs and FSAs. Plan details vary, so save your receipt and check with your benefits or health provider for eligibility.






.png)
.png)

